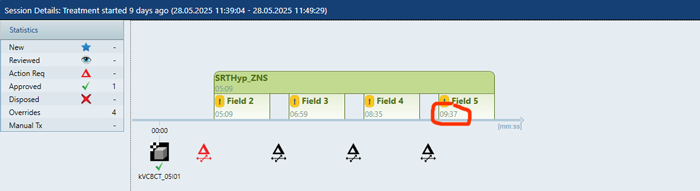
A Clinical HyperArc Case with 45 Targets
HyperArc™ is a fascinating method of precision radiotherapy for the TrueBeam. It is intended for radiation therapy in the brain and is limited to this body region, as VMAT arcs are delivered with large table rotation angles (90°-270°), which would not be possible in other body regions.
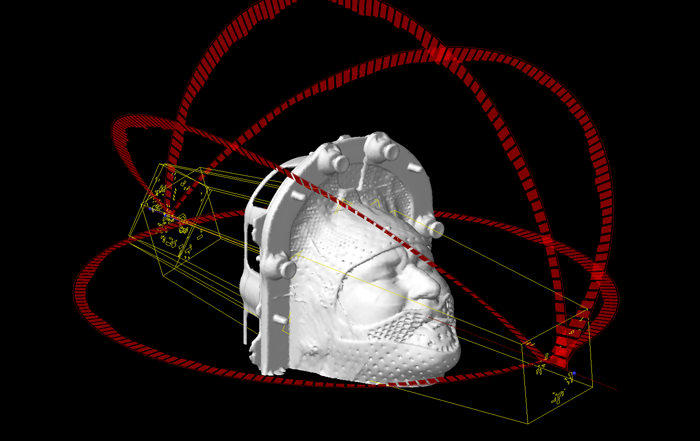
(Field arrangement of the clinical HyperArc case.)
A special head support approved by Varian (Encompass™ from QFix) must be used to immobilize the patient. This consists of a carbon base plate and two mask halves. The mask material is stronger than that of standard head/neck masks. Furthermore, the patient bites into a bite block integrated into the mask, which provides additional stability.
HyperArc reveals its full potential if multiple brain metastases are to be treated. The total duration of a treatment session is independent of the number of targets, since all targets are treated simultaneously. This is a fundamental difference from other methods such as the Elekta Gamma Knife®, where the targets are treated one after the other1.
With HyperArc, a complete session, from the CBCT to the delivery of the last MU, typically takes less than 10 minutes (the first day treatment may take a little longer, but this is the case with all techniques). Several factors make this possible. One is automation: between the fields, while the couch moves, the beam is on "hold", which alone saves 18 seconds per field for the cal/check-cycle. Another factor is the use of the FFF energy.
Our institute started to use HyperArc2 clinically in 2021 (see our introduction to the technology). What has changed since then? What has stayed the same?
First, the things that have remained the same:
- The technical planning process, as described in the introduction, is unchanged.
- In Contouring, thorough preparation for optimization is a good investment, especially if many targets are to be treated.
- The treatment itself is still more "worry-free" than with other techniques. The only additional effort compared to coplanar VMAT treatments is that you have to check whether there is enough free space around the treatment table for the large couch rotations. All objects in this area should be removed, for instance, a pair of shoes ...
What has changed since 2021?
- On the CT (now: goOpen Pro), imaging is performed with 1 mm slice thickness using the Precision Matrix (1024 x 1024 px). Due to the larger data volume, care must be taken to ensure that the scan area is not extended unnecessarily. Otherwise, memory problems could arise during optimization.
- On the CT, CARE kV determines the optimal tube voltage. Eclipse is calibrated for all tube voltages between 70 kV and 140 kV with regard to the conversion of HU to mass density (AcurosXB), so any CT dataset can be used directly for dose calculation. It is not necessary to generate two reconstructions (one for contouring and one for dose calculation).
- To achieve the optimal dose distribution in each individual target, non-overlapping shell-shaped auxiliary structures are typically created for optimization (described in detail below).
- Dosimetric verification before the first treatment is no longer performed by measurement (Varian Portal Dosimetry or EPIQA) but by 3D Monte Carlo calculation (VERIQA). For HyperArc, we apply a gamma test with 2%/1mm and also check the mean dose deviation in each individual target.
Clinical Example
A male patient with multiple brain metastases (melanoma) was assigned to stereotactic treatment. Eleven targets were identified for treatment. The dose prescription was 5 x 6 Gy to the 80% isodose (30 Gy at the border of the PTVs, 37.5 Gy in the center). Treatment was scheduled every other day.
Imaging and Contouring
After fitting the HyperArc mask, the planning CT scan was done with the following settings: 120 kV, 600 mm sFOV, Precision Matrix, 257 slices with 1 mm slice thickness, iMAR metal artifact reduction.
After registration with MR images, contouring was performed in Eclipse (GTV, CTV = GTV + 1 mm, PTV = CTV + 2 mm). The size of 10 out of the the 11 GTVs ranged between 0.3 cm3 and 2.0 cm3. Only one GTV, GTV05, was much larger (13.1 cm3).
Due to the large number of targets, they were no longer labeled with "medical location information" (frontal, lateral, etc.), but simply numbered consecutively (e.g., PTV01 – PTV11).
Subsequently, auxiliary (helper) structures were created during contouring. For example, with 01a = PTV01 – CTV01, 01b = CTV01 – GTV01, 01c = GTV01, a group of three non-overlapping shell-shaped structures (01a, 01b, 01c) was created. This was repeated for all PTVs, which resulted in 3 x 11 = 33 additional targets, to which we assigned the volume type "PTV." (Such processes would be very suitable for Eclipse scripting!)
Another target structure, PTVtotal (or PTVZNSgesamt), corresponds to the sum of all PTVs and serves as the plan target volume (every Eclipse plan formally requires a planning target volume).
In total, the structure set therefore contained 45 targets: 01a,b,c to 11a,b,c (33), PTV01 to PTV11 (11), PTVtotal. Now we were ready for optimization.
Optimization and Evaluation
The HyperArc optimization, as described in the introduction, works slightly differently than normal VMAT or IMRT optimizations, as a special wizard is started up via "New HyperArc Plan...". Here, we select all 45 PTVs, but not the CTVs and GTVs.
By making this selection, Eclipse receives the necessary (geometric) information for the field arrangement. The arcs around Cosmo's head give information which arcs will later be used in the plan. See next image.
When newly selected, each target is initially assigned the plan's total prescribed dose (30 Gy). We change the values in this dialog because we know that all "a" targets are the outermost shells, while the "c" targets correspond to the inner GTVs (the "b" shells are in between). To ensure that the enclosing isodose will be 30 Gy, while the centers receive 37.5 Gy, we increase target dose for the "b" and "c" targets:
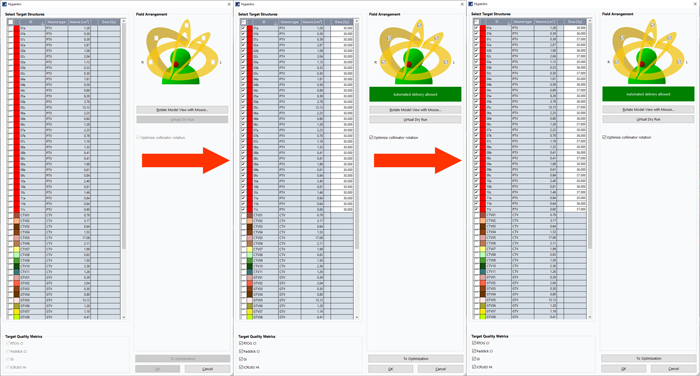
(Selecting targets and modifying target dose. The 11 PTVs are at the end of the list (off screen)).
All "b" targets should receive at least 36 Gy, and all "c" targets 37 Gy. This ensures that the target doses are maintained should it become necessary to modify the plan using "Modify HyperArc Plan...". Otherwise, all targets would be reset to 30 Gy after a modification.
We can now start the PO using the "To Optimization" button (current version is 18.1).
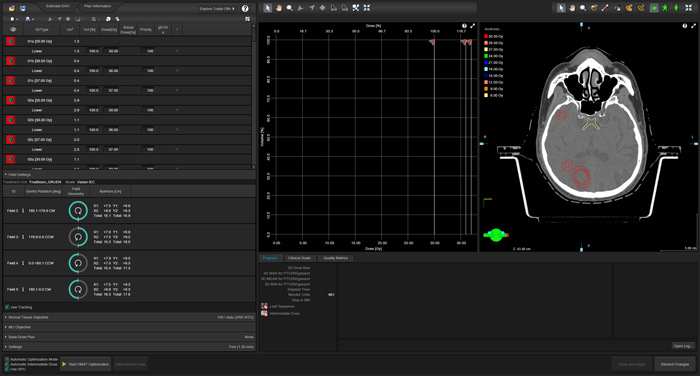
(The "virgin" PO after presssing "To Optimization").
Varian assumes that targets in HyperArc normally only receive lower objectives, but no upper objectives. Therefore, a (long) list of targets appears on the left, which requires a lot of work if we want to change this.
We want to add an upper objective to each of the 33 shell targets. The "a" targets receive 36 Gy as an upper objective, the "b" targets 37 Gy, and the "c" targets 37.5 Gy.
We set all target priorities to 100 (the same priority value as for the Normal Tissue Objective, SRS-NTO). This step, although somewhat tedious with a high target count, would also be ideally suited for Eclipse scripting. For now, the non-overlapping targets PTV01 – PTV11 and PTVtotal receive a priority of 1 for their upper objectives, the lowest possible value, but they could also get "relevant" priorities should is become necessary to "shape" the overall DVH curve of a single PTV during optimization. Therefore it is better to keep the PTVs with the "target" status. If we decide to add another target at a later stage, the HyperArc plan has to be "officially modified" (via "Modify HyperArc Plan ..."), which means extra work, as already mentioned.
All CTVs and GTVs can be removed by using “Exclude structures from optimization”.
What about the organs at risk?
In this specific example, only the brainstem comes close to a few targets, but normally the very powerful SRS-NTO alone ensures that the dose decreases as quickly as possible outside of the targets. In addition, the very low transmission of our HD120-MLC ensures that the rest of the brain receives the lowest possible dose3,4.
All other structures not used for optimization, such as brain, skin, etc., are excluded from optimization.
The use of the Precision Matrix, the large number of structures, and the selection of "Fine (1.25 mm)" as the structure resolution mean that it takes approximately four minutes for the first dose distributions and DVH curves to appear on the screen. After that, however, progress is rapid. One minute after the start of optimization, the sets of curves for the a, b, and c targets have already largely converged (red curves):
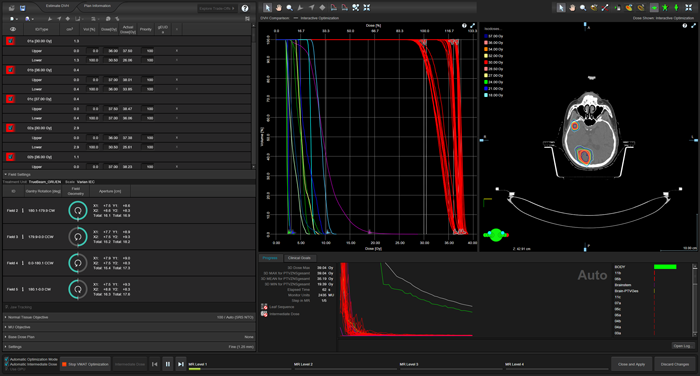
(Optimization after 62 seconds.)
And here another snapshot a little later:
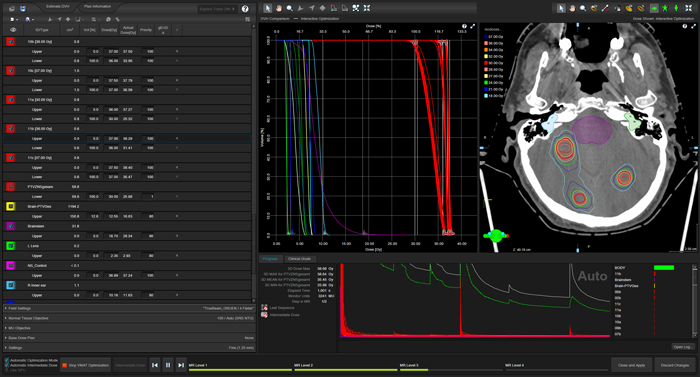
(Optimization after 1001 seconds.)
Due to the different volumes of the targets and the fact that some of them are isolated in the brain, while others are in close proximity to other targets, the sets of curves cannot perfectly overlap. Ultimately, the dose coverage at the PTV border will be relevant for the assessment.
In the example, a completely new optimization cycle, in which all optimization steps were completed (MR Level 1-4, intermediate dose, MR Level 3-4, final dose calculation), took 52 minutes (all steps with maximum resolution, as described here). This is a fantastic speed, considering the use of the Precision Matrix and the maximum resolution in all steps.
An optimization cycle may be run multiple times, as different plan variants are usually created and compared. However, in this case, an existing optimization is continued from MR Level 3 instead of starting from scratch, which is much faster.
By adjusting the Plan Normalization Value, we then normalized the example plan to 37.5 Gy at 3D dose maximum. Now dose is fixed. Next step is to check dose coverage at the border of all targets.
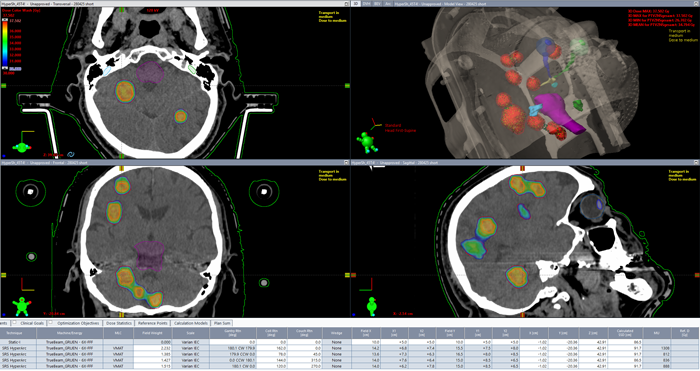
(Final dose distribution. Dose color wash starts at 30 Gy.)
We generally require that the dose coverage, i.e., the V100% [%], be between 95% and 98% for each target. In addition to a visual check in all slices (dose color wash from 30 Gy upwards), Eclipse can automatically calculate and display this value for all structures. In this specific example, the V100% value for the final plan was between 98.6% and 99.6%.
Dose Verification and Treatment
As already mentioned, one of the changes to the clinical workflow since 2021 is that the dosimetric verification of the approved plan before the first treatment is now performed with 3D-Monte Carlo calculation instead of Portal Dosimetry measurement.
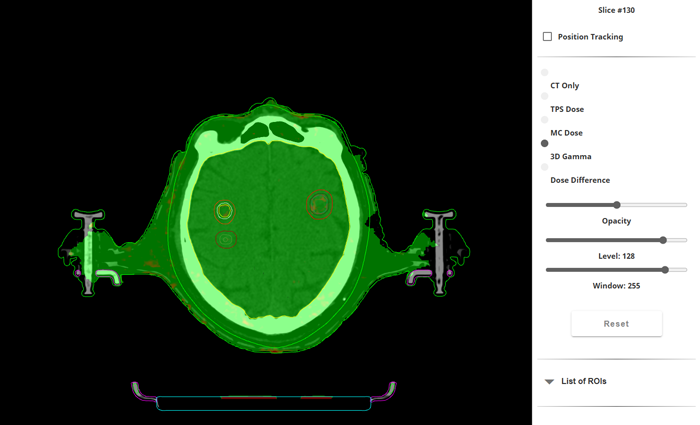
(VERIQA Gamma map for 2%/1mm.)
We have set up the decision trees for the VERIQA evaluation templates so that if the plan name begins with "SRT", "SBRT" or "Hyper," VERIQA automatically uses a template that performs a tighter gamma test than usual, a DVH evaluation, and a comparison of the mean dose of all targets.
In this specific example, the 2%/1 mm gamma pass rate was 99.96% (5% threshold). Mean dose deviation for the PTVs was between -0.6% and -2.08%, the minus sign meaning that MC doses were slightly smaller.
Treatment of the HyperArc plan, as already mentioned, is the fun part. After aquiring a CBCT which is mandatory, the online match result is used to apply 6 degrees of freedom couch shifts with the PerfectPitch couch (just like all other 3D-planned treatments).
The first session took a little longer (17 minutes), but sessions 2 - 5 took well below 10 minutes:
This is the biggest "asset" of HyperArc treatments. Regarding the mechanical machine performance (isocenter size etc.), we rely on the Machine Performance Check (MPC) from Varian with its enhancement for large couch rotations, which is performed every morning.
Literature
1For 53 metastases, Rebecca F. Krc et al. reported a delivery time of 6.5 minutes (HyperArc) vs. 412 minutes (Gamma Knife).
2 An early article in Nature from the same year (2021) compares the dosimetric performance of HyperArc, RapidArc (the standard Varian VMAT technique) and CyberKnife (G4 model).
3 A dosimetric comparison of HyperArc-VMAT, Manual-VMAT, Brainlab Elements and Gamma Knife can be found in: Vergalasova I, Liu H, Alonso-Basanta M, Dong L, Li J, Nie K, Shi W, Teo BK, Yu Y, Yue NJ, Zou W, Li T. Multi-Institutional Dosimetric Evaluation of Modern Day Stereotactic Radiosurgery (SRS) Treatment Options for Multiple Brain Metastases. Front Oncol. 2019 Jun 7;9:483. doi: 10.3389/fonc.2019.00483. PMID: 31231614; PMCID: PMC6568036.
4 Hiscoke K, Leong A, Hogan AM, Cowley I. Plan quality assessment of modern radiosurgery technologies in the treatment of multiple brain metastases. Biomed Phys Eng Express. 2024 Feb 5;10(2). doi: 10.1088/2057-1976/ad218f. PMID: 38262047.